Lämpötilayksikkömuunnin
Syötä alla olevan lämpötilayksikkömuuntimen ”Arvo” (Value) -kenttään lämpötila ja valitse lämpötilayksikkö ”Yksikkö” (Unit)-valikosta. Paina sitten ”Muunna” (Convert) -painiketta ja katso tulokset alla olevasta taulukosta. Käytä luvussa desimaalipistettä (ei pilkkua).
| Value | Unit |
|---|---|
| °C | |
| °F | |
| K | |
| °Ra | |
| °Re |
Kuinka muunnat celsiukset fahrenheiteiksi ja toisin päin helposti päässälaskuna?
Kuinka muunnat celsiusasteet fahrenheitasteiksi helposti päässälaskuna?
Saatat joskus joutua tilanteeseen, jossa sinun pitää muuttaa celsiusasteet fahrenheitasteiksi, eikä käytettävissäsi ole yksikkömuunninta. Ei hätää, muuntaminen ei ole kovin monimutkaista, kunhan noudatat seuraavaa ohjetta.
Muuntaminen tehdään kahdessa vaiheessa:
Vaihe 1: Yksi celsiusaste on 1,8 °F (tai oikeastaan 9/5, mutta 1,8 on helpompi ymmärtää). Luvulla 1,8 kertominen päässälaskuna ei ole kovin helppoa, mutta tähän on kätevä ratkaisu: kerro celsiusasteet ensin kahdella (helppoa) ja vähennä sitten tuloksesta 10 % (todella helppoa).
Vaihe 2: Muista, että 0 °C on 32 °F. Älä unohda tätä! Lisää saamaasi tulokseen 32 °F.
Tässä muutamia esimerkkejä:
Kuinka monta fahrenheitastetta on 20 °C?
- Kerro 20 kahdella eli 20 x 2 = 40.
- Vähennä tuloksesta 10 % eli 40 – 4 = 36.
- Lisää 32 °F eli 36 + 32 °F = 68 °F.
- Näin ollen 20 °C = 68 °F. Aika helppoa, vai mitä?
Kuinka monta fahrenheitastetta on 100 °C?
- Kerro 100 kahdella eli 100 x 2 = 200.
- Vähennä tuloksesta 10 % eli 200 – 20 = 180.
- Lisää 32 °F eli 180 + 32 °F = 212 °F.
- Näin ollen 100 °C = 212 °F.
Kuinka monta fahrenheitastetta on 0 °C?
- Oikea vastaus on 32 °F, eli sama lukema, joka sinun piti alussa painaa mieleesi.
Jos haluat helpottaa päässälaskua, kerro celsiusasteet kahdella ja lisää 32 °F. Tällöin lopputulos on kuitenkin hieman liian suuri, joten sinun pitää pienentää tulosta hieman. Edellisessä esimerkissä (20 °C) laskukaava olisi 20 x 2 + 32 = 72 °F. Tämä laskutapa antaa siis vain karkean arvion, ja tulos on hieman liian suuri
Kuinka muunnat fahrenheitasteet celsiusasteiksi päässälaskuna?
Fahrenheitasteiden muuntaminen celsiusasteiksi:
Vaihe 1: Vähennä fahrenheitasteista 32 °F.
Vaihe 2: Kerro fahrenheitasteet 5/9:llä. Siis mitä? Nyt taitaa mennä liian vaikeaksi, koska 5/9 on 0,555555. Saat kuitenkin melko tarkan tuloksen, jos jaat arvon kahdella ja lisäät sitten 10 % (oikeastaan pitäisi lisätä 11,11 %).
Esimerkkejä:
Kuinka monta celsiusastetta on 100 °F?
- Vähennä 32 °F => 100 – 32 = 68.
- Jaa 68 kahdella => 68 : 2 = 34.
- Lisää 10 % => 34 + 3,4 = 37,4.
- Näin ollen 100 °F on noin 37,4 °C.
Lopputulos on tarkempi, jos 10 %:n sijaan lisäätkin 11 %. Käytännössä helpointa on lisätä ensin 10 % ja sitten erikseen vielä 1 %. Katsotaanpa siis edellistä esimerkkiä uudestaan:
Kuinka monta celsiusastetta on 100 °F?
- Vähennä 32 °F => 100 – 32 = 68.
- Jaa 68 kahdella => 68 : 2 = 34.
- Lisää 10 % => 34 + 3,4 = 37,4.
- Lisää vielä 1 % => 37,4 + 0,374 = 37,77 °C. Kun lopputulos pyöristetään ylöspäin, vastaukseksi saadaan 100 °F = 37,8 °C.
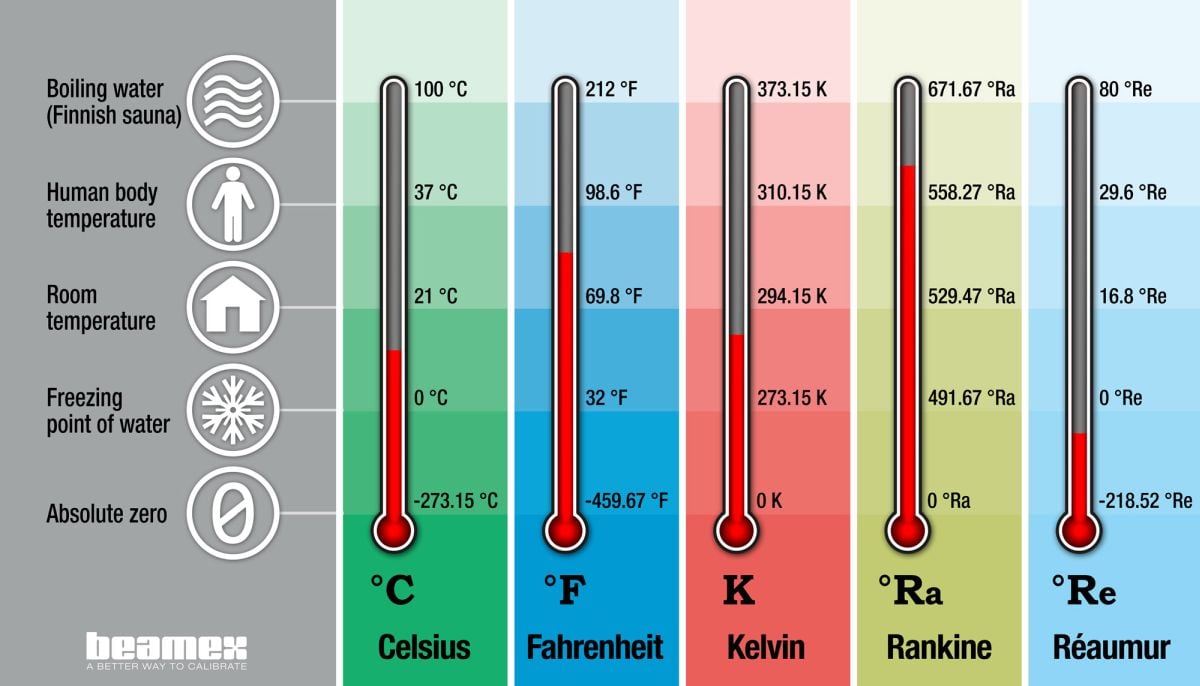
Lämpötila ja lämpötilayksiköt
Mitä lämpötilalla tarkoitetaan?
Lämpötila on aineen energiatilaa kuvaava intensiivinen suure. Kaikki materiaalit sisältävät atomeja ja molekyylejä, jotka liikkuvat, värähtelevät tai pyörähtelevät jatkuvasti. Kappaleen lämpötila voidaan määrittää sen atomien ja molekyylien keskimääräisen kineettisen energian avulla.
Mikä on kelvin (K)?
Kelvin on kansainvälisen SI-järjestelmän mukainen lämpötilan mittauksen perusyksikkö.
Kelvinin lyhenne on K (ilman astetta tai astemerkkiä). Kelvin-yksikön esitteli ensimmäisen kerran brittiläinen fyysikko William Thomson eli lordi Kelvin vuonna 1848. Vuonna 2019 tehdyn SI-järjestelmän uudelleenmäärittelyn myötä kelvinin määritelmän perustana on Boltzmannin vakion k kiinteä lukuarvo 1,380649 × 10-23 J⋅K−1.
Mikä on Celsius (°C)?
Celsiusaste on nykyisin SI-järjestelmän mukainen lämpötilan johdannaisyksikkö, kun taas kelvin on perusyksikkö. Yksikön ja varsinaisen Celsius-asteikon esitteli ensimmäisen kerran ruotsalainen tähtitieteilijä Anders Celsius vuonna 1742. Celsius-asteikon kaksi tärkeintä referenssipistettä ovat veden jäätymispiste (tai jään sulamispiste) ja veden kiehumispiste, joille on annettu arvot 0 °C ja 100 °C.
Mikä on Fahrenheit (°F)?
Fahrenheit-asteikon esitteli ensimmäisen kerran hollantilainen fyysikko Gabriel Fahrenheit vuonna 1724. Asteikon kaksi tärkeintä referenssipistettä ovat veden jäätymispiste 32 °F ja ihmisen ruumiinlämpö 96 °F. Nykyään Fahrenheit-asteikko on määritelty uudelleen niin, että jään sulamispiste on täsmälleen 32 °F ja veden kiehumispiste tasan 212 °F. Tarkistetussa asteikossa ihmisen ruumiinlämpö on noin 98 °F.
Mikä on Rankine (°R, °Ra)?
Rankine-asteikon esitteli skotlantilainen insinööri ja fyysikko William Rankine vuonna 1859. Kelvin-asteikon tavoin myös Rankine-asteikon referenssipisteenä on absoluuttinen nollapiste, 0 °Ra. Rankine-asteikon asteväli on sama kuin Fahrenheit-asteikolla, joten veden jäätymispiste on 491,67 °Ra.
Mikä on Réaumur (°Ré, °Re)? Réaumur-asteikon esitteli ranskalainen matemaatikko ja fyysikko Réne de Réaumur vuonna 1730. Sen kaksi referenssipistettä ovat veden jäätymispiste 0 °Re ja veden kiehumispiste 80 °Re.